In today’s dynamic pharmaceutical industry, Market Authorization Holders (MAHs) face increasing pressure to ensure the authenticity, safety, and full traceability of every product released to the market. A robust Pharma MAH Track and Trace solution is essential—not only for meeting stringent regulatory requirements but also for enhancing supply chain visibility, preventing counterfeiting, and ultimately safeguarding patient safety. Hicof’s high‑performance machines and software, combined with the innovative AMACENA Software, deliver an integrated, cloud‑based solution designed specifically for MAHs.
Market Authorization Holders carry the critical responsibility of overseeing the lifecycle of pharmaceutical products—from manufacturing and packaging to distribution and dispensing. In an era where counterfeiting is a global threat and regulations are continually evolving, MAHs must employ advanced technologies to guarantee the integrity of every unit.
Pharma MAH Track & Trace systems provide end‑to‑end visibility over the entire supply chain. Hicof’s solution is built on powerful, high‑performance machines and software that work in perfect harmony. Designed and manufactured with Swiss engineering excellence, our system integrates every aspect of serialization without the complexity of interfacing separate components. The result is a solution that not only meets but exceeds the demands of modern pharmaceutical regulation.
Regulatory bodies across the globe—including the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA)—demand rigorous serialization and track & trace measures. A compliant Pharma MAH Track & Trace solution ensures:
A sophisticated track & trace system is a key defense against counterfeit drugs and unauthorized distribution. With real‑time monitoring and data integration, MAHs gain:
Beyond compliance and security, a comprehensive Pharma MAH Track and Trace system drives operational excellence:
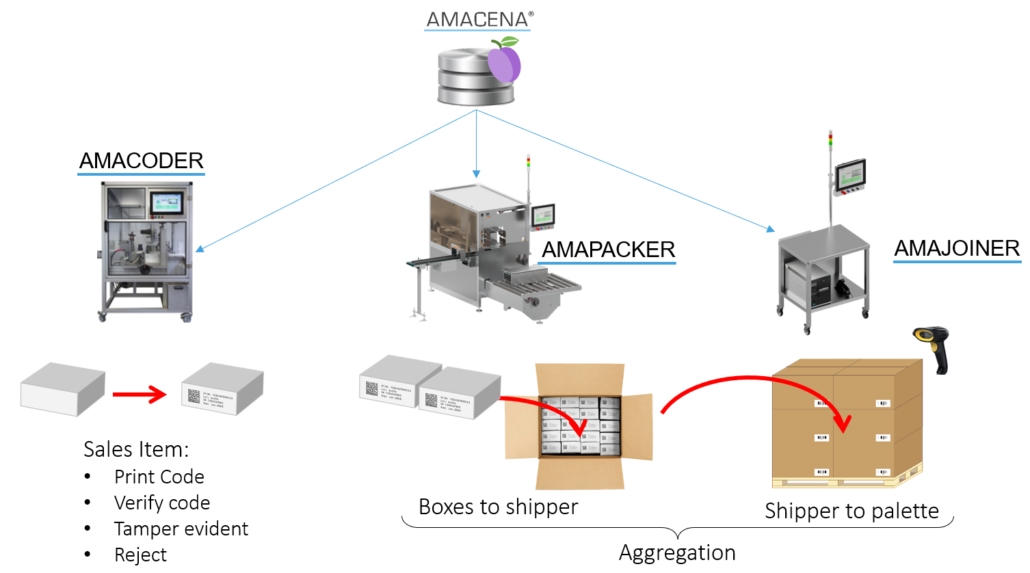
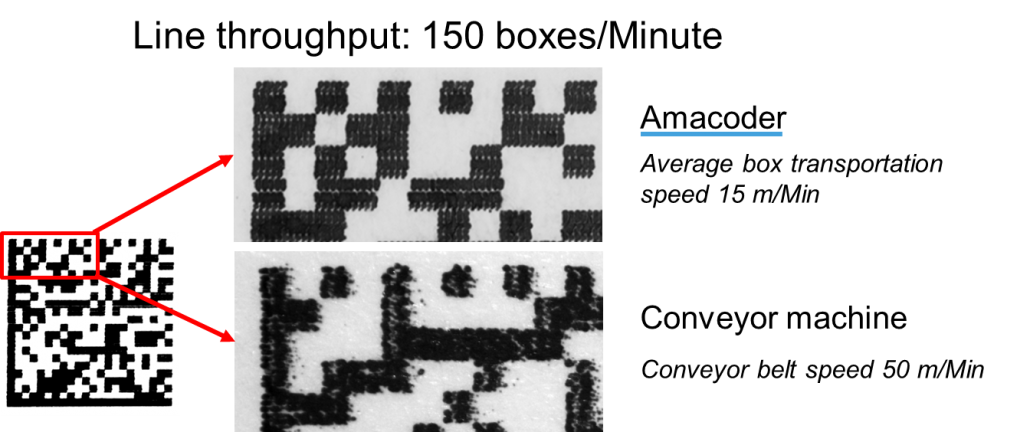
The Amacoder delivers a very high coding quality as shown in the image.
The reason for this high coding quality is as follows:
Hicof is recognized for its cutting‑edge solutions in pharmaceutical serialization. Our high‑performance machines and software are designed to work together seamlessly, providing Market Authorization Holders with unmatched reliability and efficiency.
Unlike solutions that rely on disparate systems, Hicof’s machines and software are developed in-house. This integrated approach means:
Every element of our system is 100% designed and built in Switzerland—a country renowned for its precision engineering and rigorous quality standards. This commitment to Swiss manufacturing ensures that:
At the core of our Pharma MAH Track & Trace solution is the innovative AMACENA Software — a cloud‑based platform designed to revolutionize how serialization data is managed and utilized.
AMACENA is built from the ground up for the cloud, offering a host of benefits:
One of AMACENA’s standout features is its robust 4‑eye principle change process. This critical security measure requires any changes to serialization‑relevant data to be reviewed and approved by two authorized users. Benefits include:
User experience is a top priority for AMACENA:
Implementing a comprehensive Pharma MAH Track & Trace solution like Hicof’s brings a multitude of advantages for MAHs:

Hicof AMACENA features a build-in web based label creator to create and adjust all print layouts for AMACODERS, and all connected tertiary label printers. The label creator allows GMP compliant change control of all label parameters across the enterprise.
To fully leverage the benefits of Hicof’s Pharma MAH Track & Trace solution, a structured implementation process is key. Here’s a step‑by‑step guide to ensure successful deployment:
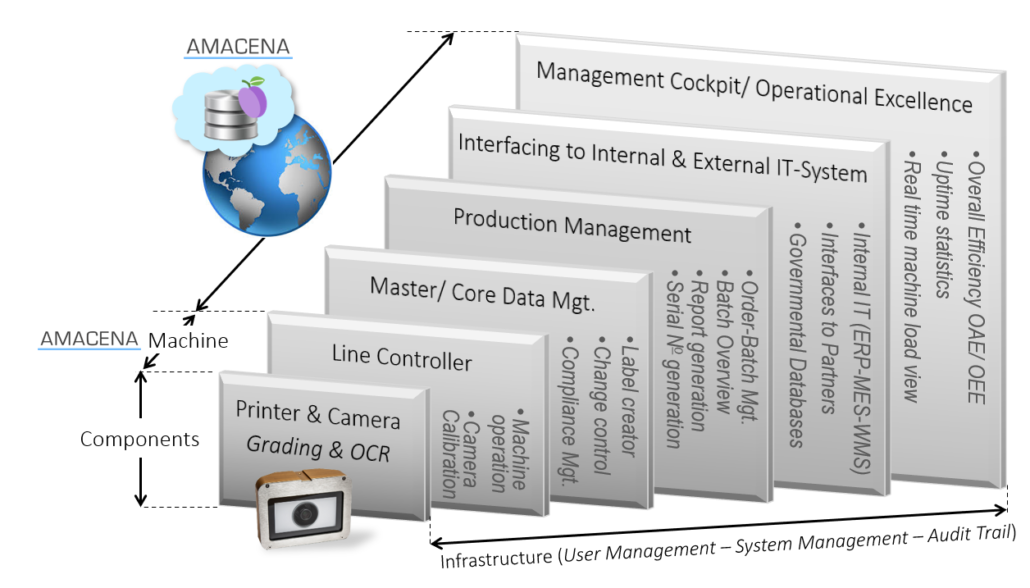
The HICOF Machines offer in combination with AMACENA an integrated IT-backbone, starting at machine level from the devices as printers, cameras and sensors up to data interfaces of governmental servers. In case of an issue, our experts have instantaneously access through remote access to all relevant parameters across all IT-servers down to the PLC, allowing a seamless and immediate support. This is the opposite of the conventional ISA-95 environment with several software and machine suppliers in which each service engineer has only access to his fraction of data, making a comprehensive root cause analysis extremely cumbersome as he has to synchronize with other involved vendors.
Hicof solves the issue by offering
One System, One Supplier, One Responsibility!

